
Figure 2-3: Nutrients cycling through a waterway ecosystem
Nutrient cycling
| Nutrients are chemicals which animals and plants need to grow. |
The chemistry of waterways is complex. There are many chemical elements and compounds present. These chemicals are involved in a multitude of ways with the biochemistry of the living things in a waterway environment. Humans complicate this chemistry by adding nutrients, trace metals and other materials which either alter the natural system or produce toxic conditions.
Important chemicals in the waterways environments include nutrients. Nutrients are vital to the whole web of life in these environments. They are required by all plants, and animal life is supported by the plants. A supply of nutrients added to the system by natural processes (from run off, rainfall or from the ocean), replaces losses and keeps the system functioning at its best.
Plants and animals require at least 30-40 nutrients for their growth and development. The most common ones are nitrogen, calcium, phosphorus, potassium, sulphur, chlorine, sodium, magnesium, iron, manganese, copper, iodine, cobalt, zinc, boron, vanadium and molybdenum. The major plant nutrients in the waterway environment are nitrate and phosphate. The nitrogen content of plant tissue is much higher than the phosphorous content.

Figure 2-3: Nutrients cycling through a waterway ecosystem
The cycling of the elements nitrogen and phosphorous from non-living surroundings through organisms and back again is called the nitrogen and phosphorous cycles. Their cycling is important to the chemical and biological makeup of a waterway's environment. Nitrogen and phosphorous are vital parts of the molecules of chemical compounds which make up living tissue.
Nutrients are constantly being removed from, or added to, the waterway environment by natural and artificial processes. On land, nutrients are returned every year to the soil by leaves, litter, roots, animal excreta and the bodies of dead animals. All these things decompose and release the nutrients into the soil, water and air. The nutrients are then taken up, first by plants, and then by animals which eat the plants, and so on through the food chain. In freshwater, estuarine and marine ecosystems, the remains of plants and animals drift to the bottom, where they decompose. The nutrients are moved to the upper layers by the circulation of the water.
Materials flow from the living, to the non-living, and back to the living in the ecosystem in a constant cycle. By means of these cycles, plants and animals get the nutrients that they need for growth. Plants and animals need some of the elements in relatively large quantities (macronutrients). Other elements (the trace elements, or micronutrients) are needed in lesser, often only tiny, quantities. Without them, however, plants and animals will die, just as they would if they lacked one of the major nutrients. However, too much micronutrient can be toxic.
| For more information about nutrients as pollution section 4.4 - Parameters: nutrients |
Sometimes the nitrogen stays bound to the soil and the speed of the water carries the soil particles tumbling and rolling into the estuary. When the water slows down, the soil particles fall to the bottom of the estuary to become part of the estuary sediments. When the sediments are disturbed the nutrients are released into the water.
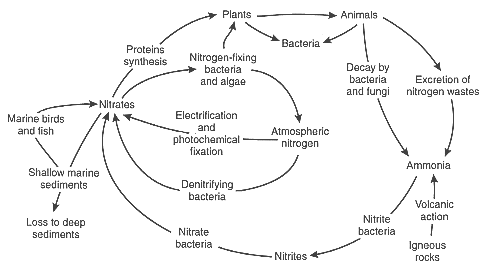
Figure 2-4: The nitrogen cycle
Adding a lot of commercial fertilisers (which contain nitrogen) to crop land increases the amount of nitrogen in the soil, and some of this can make its way into the estuary, increasing the nutrients in the waterway.
Some plants do take nitrogen from the air and form different types of nitrogen `compounds' (nitrogen mixed with other chemicals). This process is called `nitrogen fixation'. Blue-green algae carries out nitrogen fixation, and some soils contain nitrogen-fixing bacteria. These bacteria increase the amount of nitrogen compounds in the soil. Nitrogen fixation can increase by a large amount the nitrogen circulating through a community of living things.
Phosphorus comes from rock and natural phosphate deposits as a result of weathering, leaching, erosion, and by mining for agricultural use. It passes through land (terrestrial) and water (aquatic) ecosystems by way of plants, grazing animals, predators and parasites; and it is returned to the ecosystem by excretion, death and decay. Some of the phosphorus of terrestrial ecosystems escapes to lakes and seas.
Human activities have altered the phosphorus cycle. Because vegetation cropping depletes the natural supply of phosphorus in the soil (the natural phosphorous cycle is completely altered), phosphate fertilisers must be added. Part of the phosphorus used as fertiliser is removed in harvested crops. These crops are then transported away from where they are grown and the phosphorus isn't put back into the soil. However, the phosphorus in these harvested crops is eventually released as waste when foodstuffs made from the crops are processed or eaten.
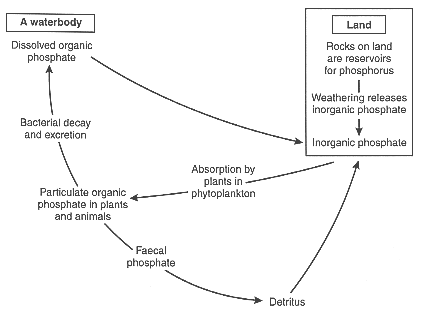
Figure 2-5: The phosphorous cycle
There are several ways human activities add phosphorus to the waterways. Firstly, there's a lot of phosphorus in the waste from food processing plants and in animal feedlots. The phosphorus gets into the waterways through drains and animal excreta. Secondly, some of the phosphate fertiliser from agricultural land may be washed into drains and creeks and then get into the estuary. How much ends up in the estuary depends on the soil type (different soils hold more phosphorus than others) and the rainfall (short bursts of heavy rain can wash more soil into waterways than a long period of gentle rain).Thirdly, large quantities of phosphates also come from urban areas, especially from factories, sewerage systems and run off from lawns and gardens. Secondary sewage treatment, which is the system most widely used in Western Australia, is only 30% effective in removing phosphorus. The remaining 70% of the phosphorous stays in the effluent and is discharged into the natural environment. In estuaries, the phosphorus is taken up rapidly by the plants, resulting in a great increase in the number of aquatic plants.
| For more information about nutrients as pollution section 4.4 - Parameters: nutrients |
Phosphorous and nitrogen are the two most important nutrients. Although plant growth requires carbon, hydrogen and oxygen as well as 19 other elements, it is high concentrations of nitrogen and phosphorous that create the blooms of algae and aquatic plants which form the base of the food chain. These are the two nutrients that are used to fertilise crops and lawns.
The severity of eutrophication and its effects on a waterway depend on the physical, chemical and biological characteristics of the waterway. For example, the Peel-Harvey estuarine system before the Dawesville Channel was put in and created a flush, was an example of a poorly flushed system. Excessive nutrients resulted in the growth of green algae Cladophora. This alage accumulated on the beaches where it decomposed and released hydrogen sulphide and other noxious odours. As well, the Peel-Harvey system also had blooms of the microscopic blue-green algae Nodularia, which clogged the water, taking up much of the space normally available to other living things. This resulted in deoxygenation and fish being killed. The Dawesville Channel, which was constructed in 1994, is designed to help flush nutrients from the system, so that the cycle of noxious algal blooms can stop.
In contrast, Princess Royal Harbour and Oyster Harbour in Albany are relatively well-flushed waterbodies. However, macroalgae species have increased because of the high levels of nutrients in these waterways. The algae was harvested to try and stop it smothering the seagrass beds.
Soils are made by the mechanical and chemical breakdown of rocks, mixed with the remains of decayed plants and animals. These become a soil's nutrients. Rainwater percolates through the soil and dissolves these nutrients, taking them to the groundwater, or a nearby stream, and then into another waterway. How much nutrient remains in the soil after rain depends on the types of plants and soils in the region.
Agriculture
To grow more and more food, more and more fertiliser is used in agriculture. It's common that the amount of fertiliser applied to the soil is higher than the amount that the soil can hold. When it rains, a lot of the nutrients in the fertiliser are washed into waterways. How much nutrient washes off agricultural land depends on how long and how heavy it rains, the slope of the land, the soil type, the sorts of plants being grown on and near the farm, the type of agriculture, and the condition of the waterway's fringing vegetation.
Agriculture that uses a lot of nutrients (such as growing vegetables or pigs) is increasing in most catchments in south-western Western Australia.

Vineyard on the Swan River
These sorts of activities either use a lot of nutrients or produce waste containing large amounts.
Catchment clearing
Clearing vegetation from the catchment increases the amounts of run off and sediment entering waterways. The native plants which would normally have used the soil's nutrients, taken up a lot of the water, and bound the soil together with their deep roots, have been removed. This has increased the amount of water run off and sediment carrying nitrogen and phosphorous that reaches waterways.
Septic tanks
A great deal of domestic water goes into septic tanks. This water is rich in nutrients from human waste, household waste and detergents. Not much is known about how much nutrient enters waterways from septic tanks, but it is probably a lot.
Stormwater
Untreated urban stormwater may help to increase eutrophication. Stormwater contains significant quantities of nitrogen and phosphorous from gardens, industrial debris and street-cleaning practices.
| For more information about nutrients as pollution section 4.4 - Parameters: nutrients |