Water quality parameters
| Things which can be measured in some way or another, to see if there is change, are called 'parameters'. Water quality parameters are things which can be measured to find out the quality of the water in a waterbody. |
Measurement of these parameters is undertaken by the Water and Rivers Commission, local waterways management authorities, local governments, local school groups, industries and farmers to help understand water quality changes. Monitoring water quality can provide a good indicator or whether waterways and catchment management initiatives are effective.
| Salinity is the total concentration of salts in water. |
The two most common constituents of the salts in water are the chloride ion and the sodium ion. The worldwide average salinity of sea water is 35 000 parts per million (ppm) and fresh water is generally considered to be less than 5000 ppm. The salinity of waterways may vary from fresh to hypersaline (salinity levels greater than sea water). (See Table 4-2 below.)
| Salinity | Uses |
|---|---|
| 0-480 ppm |
|
| 480-1500 ppm |
|
| 1500-6000 ppm |
|
| Over 6000 ppm |
|
Different amounts of salt in the water in different mixtures (called 'salinity regimes') exist in waterways, particularly in estuaries. An estuary is a region where fresh water mixes with sea water. The two waters are of very different chemical compositions. The density of sea water is much greater than that of fresh. The two do not mix and tend to act as separate bodies of water. Sea water, being heavier, sinks to the bottom. This results in layering of the water and is known as stratification. Mixing will only occur if some force is applied, such as wind, to the water surface or current flow. Because of the great variations of freshwater inflow into rivers in south-western Western Australia salinity regimes vary at different times of the year.
The estuaries of south-western Western Australia are unusual because many of the rivers only flow in winter. This causes the salinity of the estuary to differ dramatically between summer and winter. In winter the salinities tend to be lower than in summer because there is more freshwater run off. At this time of the year the freshwater inflow to the estuary is often strong enough to prevent or reduce sea water penetrating into the system on the incoming tide.
When run off decreases in spring, sea water is 'pumped' into the estuary by the tides and moves upstream beneath the outflowing fresh water. The salt water moves under the fresh water because the salt water is heavier and denser. The two waters are gradually mixed by wind in the open water of the estuary and the salinity of the surface water increases. Run off continues to decrease into summer and the flow of the river slows. The mixed water moves further upstream until the full summer condition is established. In summer the estuary normally has a salinity similar to that of the sea. However, evaporation from the surface further increases salinity and the estuary water is sometimes more salty than the sea. This process of layering of freshwater over sea water is called a 'salt wedge'.
Salinity levels influence what types of living things (called 'biota') are able to live in a waterway. Those biota which are adapted to high saline conditions will only survive when salinity levels increase. Salinity levels are increasing in many of the rivers and estuaries in the south-west as a result of clearing vegetation.
The layering of fresh water over sea water can result in the deeper bottom waters being isolated from the surface. This layering is a normal late winter condition (July to October). At this time little or no oxygen can get from the air through the surface water to the deeper waters. The deeper waters may then become deoxygenated because fish and other creatures use up the oxygen in these waters. Plants release oxygen into the water through a process known as photosynthesis, but it is usually at a low level at this time. Occasionally the deoxygenated deep water is pushed to the surface. Fish in the upper water might then die because there's not enough oxygen. Invertebrates (such as molluscs and worms) which live in the mud and sand on the floor of a waterway and are unable to escape to areas of oxygenated water may also die as result of the lack of oxygen. Numbers of dead animals may vary from hundreds of fish to millions of invertebrates. People can more easily see dead fish, yet the death of millions of invertebrates may be more catastrophic because these animals form the basis of many food chains in an estuary.
The salinity of a waterway is measured using a salinity meter. The higher the salinity level the higher the concentration of total salts in the water. Salinity is expressed in parts per million (ppm). Salinity levels for different uses are outlined Table 4-2.
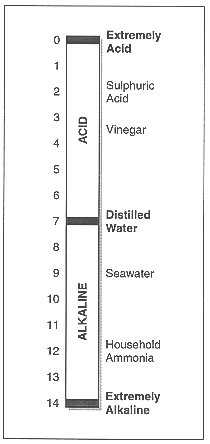
| Water can be acid or alkaline. How much one way or the other affects how that water can be used. The degree of water acidity or alkalinity is called its pH. |
The pH of a waterbody varies with the soil and rock types in surrounding areas and the sorts of substances entering the water.
 |
If pH in a waterway is out of balance, it can be harmful to creatures and plants in a waterway ecosystem. Sudden changes in pH can affect bacteria and micro-organisms which help to purify water. If pH changes from normal, plants and animals in the water may become weakened and more likely to get diseases and parasites. They may eventually die. Wildlife can also be harmed by chemicals such as cyanide, ammonia, heavy metals and chlorine. The water's pH levels can make the effects of these substances worse, or less harmful, depending on the effect of acidity or alkalinity on the particular chemical. Chemicals can also swing the pH level one way or the other, which can harm wildlife, too, even if the chemical doesn't harm them directly. Common substances that can easily disturb the natural pH of a waterway include fertilisers, industrial effluent, detergents and insecticides.
pH is measured using different sorts of meters. The meter will give a reading on a scale of 0 to 14. Neutral waters have a pH of 7. Acidic waters are below 7 and alkaline waters are above 7. The usual pH for Western Australian waters is between 5.0 and 8.5, though wide natural variations can occur in certain areas. Drinking water should fall between pH 6.5 and 8.5.
As a general rule the following pH values can be used to see if there is water pollution:
| pH 5-7 | Normal (if there is no limestone in the area) |
| pH 7-8.5 | Normal (if there is limestone in the area) |
| pH 8.5-9 and pH 4-5 | May be polluted |
| pH < 4 or > 9 | Pollution problem |
| Temperature is how hot something is. |
Another way of describing temperature is as 'a condition of a thing which determines the rate at which heat is transferred to or from it'. Because every thing, living or not, transfers heat at different speeds depending on what the thing is made up of and how it reacts with the outside, temperature is measured using an arbitrary scale. In Australia, heat is measured in degrees Centigrade (°C).
Water temperature changes with the depth of the water and the season of the year. The surface of the water is likely to be warmer than the deeper water because the surface water gets more heat from the sun. Waterways are likely to heat up in hot weather, especially if they are shallow and there's not much water flow. As the temperature rises biological activity increases: animals are more active and plants increase their growth. This is not necessarily a bad process. It is often part of the natural cycle of the system.
Increases in the temperature of a waterway may be caused by:
Sudden changes of temperature in a waterway, or a small change in temperature that goes on for a long time, can have a big effect on animals and plants in the waterway. A change in temperature affects the amount of oxygen which is dissolved in the water. If the temperature falls, the rate at which water plants can photosynthesise (turn sunlight into oxygen) and add oxygen to the water is reduced. If this happens, other creatures don't have as much oxygen and may die.
Changes in the temperature of the waterway will also affect the pH and salinity of the water.
Higher temperatures change the toxic effects of a number of substances (such as heavy metals and ammonia), but not in the same way. The harmful effects of ammonia are lessened, but the effects of a number of heavy metals become more harmful as the temperature rises.
In addition, the temperature of a waterbody affects how animals and plants reproduce. Fish need certain temperatures so they can reproduce.
The temperature of a waterway is measured using a variety of temperature meters or thermometers and is recorded in degrees Centigrade (oC). Usually, how much the temperature of the waterway varies from one area to another is measured and the difference is then calculated. For example, the temperature of a river might be measured upstream of where a creek enters the river, and then downstream of the creek's entry. The difference that is calculated between the two temperatures will be the average temperature for the river between those two points. If there is a big difference between the temperatures above and below the creek, it may mean that the creek is bringing polluted water into the river.
As a general rule the following temperature differences can be used to see if there is water pollution:
| Difference between 0°C and 2°C | Acceptable |
| Difference between 2°C and 15°C | May indicate pollution |
| Difference > 15°C | Indicates thermal pollution |
| Dissolved oxygen (DO) is the amount of oxygen in the water for plants and animals to use. |
The level of oxygen in the water fluctuates naturally during the day and night and from season to season. This is because plants photosynthesise (make oxygen from sunlight) during the day and respire (use oxygen) during the night. The wind helps to bring fresh oxygen into the water, too.
A fall in the dissolved oxygen content of a waterway is one of the first signs of pollution. This is explained further below.
For fish to be able to live, there must be enough oxygen in the water for them to use. The DO level shouldn't be less than 4.5 milligrams of oxygen per litre of water (called '60% saturation'). If it falls below 4.5 mg/L, then fish will probably die.
Sometimes, too much dissolved oxygen in water can indicate problems. A DO level greater than 100% saturation can happen when there is a lot of algae. These tiny plants take in sunlight, water and carbon dioxide and produce carbohydrates and oxygen.
How much dissolved oxygen is in the water is affected by the `biochemical oxygen demand' (BOD), the salinity and temperature of the water, how much plants are photosynthesising and respiring, how much oxygen is lost to the air, and how much is gained from it.
| Biochemical Oxygen Demand (BOD) is a way of measuring how much oxygen is needed for things to be able to decay in water. |
If the BOD is high, it often means that the DO is low. In other words, bacteria and other organisms are using up oxygen to help them break down dying or dead plants and animals in the water. If there is a lot of this going on, there's less oxygen available for fish and other creatures to use, and they may die if the oxygen level in the water falls too low.
If the dissolved oxygen level is 100% saturation, the river system is clean. If the DO level is lower than 100%, there might be a lot of bacteria decomposing plants and animals, and plants might be respiring a lot at night. Or plants may not be producing much oxygen, for example, because there aren't many plants because they've been killed off by toxins, there's reduced light or they've been physically removed by dredging. If the DO level is higher than 100%, plants might be photosynthesising more (such as during an algal bloom).
Sea water with 100% saturation usually contains 6.6 mg/L (in winter) to 9.0 mg/L (in summer) dissolved oxygen. A saturation level below 60% (4.5 mg/L) can be harmful to the living things in the sea water. There just isn't enough oygen for them to use.
When the temperature and the amount of salt in the water increases, it is harder for oxygen to be dissolved in the water (the increase reduces the 100% saturation and the DO). The effect of changing temperatures and salt levels on the amount of oxygen in the water is different from season to season.
BOD is measured by collecting a water sample and measuring the DO level in the water. The sample is then incubated for five days and the DO level measured again. The change in DO level shows how much oxygen is used up in the breakdown of organic matter.
| Suspended solids are small bits of soil and other matter in the water. |
Small things, like bits of soil, that don't dissolve in water are called 'particulate matter'. The larger particles (called 'settleable solids') tend to sink and settle on the bottom of the waterway. The smaller particles (called 'colloidal solids'. 'Colloid' comes from the ancient Greek kolla, meaning 'glue') often stay floating in the water. These suspended solids come from different places:
| Settleable solids: | sand and coarse silt particles |
| Colloidal solids: |
|
Pollution, such as toxic heavy metals, nutrients and pesticides, is easily carried by fine particles of soil in the water. The polluting materials stick to, or mix with, the solids and are carried through the waterway with them. These fine solids are also good homes for bacteria and other micro-organisms that may become pathogenic (disease-producing) to humans and animals.
Further downstream, the fine sediments settle on the bottom and block channels, fill river pools and estuaries and silt dams. The channels and dams become smaller and don't hold as much water. In winter, this can lead to flooding. If the estuaries and river pools fill up with sediment, they are no longer good homes for plants and animals.
 |
How good water is at letting light through is called 'turbidity'. How much suspended solids there are in the water, and how they reflect and scatter the light that hits them, affects the ability of the water to transmit light throughout the waterway. If there are a lot of suspended solids in the water, the turbidity is high. That is, the water is hard to see through. It looks opaque or cloudy.
The concentration of suspended sediments in water is normally expressed in milligrams of solids per litre of water (mg/L). A sample of the water from a river or other body of water is evaporated or filtered to get the particles of solids. Filtering involves drying a glass microfibre filter paper at 105oC, weighing it, then filtering a water sample through the filter paper. The paper is then dried again at 105oC and reweighed. The difference between the two weights is the weight of suspended sediment.
To measure turbidity outside a laboratory, at the river or other water body, a black and white disc known as a secchi disc is lowered into the water. The depth at which it can no longer be seen is measured. The turbidity is then said to be so many metres (or fractions of metres, depending on what depth it disappeared from sight).
| Depth disc disappears (m) | Water condition |
|---|---|
| 0.25 | very muddy (algal blooms) |
| 1.5 | much suspended matter |
| 10.0 | very clear |
| Nutrients are chemicals needed by plants and animals for growth. |
| For more information about nutrients, and nitrogen and phosphorus cycles see section 2.4 |
When excessive amounts of nutrients enter the estuary it is known as 'eutrophication' (from the ancient Greek, meaning 'well-fed'). Two nutrients that encourage eutrophication are nitrogen and phosphorus. Plants need these nutrients to grow.
Too much nitrogen and phosphorus leads to excessive growth. Nutrients may be added to a waterway for many years with no obvious ill effects. They can take a long time to build up.
Phosphorus seems to be the most important nutrient for plants in the waterways of south-western Western Australia. When rain runs off land, it carries phosphorus in various forms into waterbodies. The phosphorus comes naturally from rocks, and plant and animal wastes, and also from artificial sources, such as fertiliser on lawns and paddocks. Much of the material that is carried into a waterway with run off is in the form of very small particles. It can be soil particles, plant material, animal excreta and other substances. Run off from urban and rural areas may both be sources of phosphorus in some catchments.
Phosphorus and nitrogen, the other main nutrient, come mostly from fertilisers leaching from agriculture and horticulture, sewage and animal effluent, and urban run off from heavily fertilised parks and gardens (such as golf courses). Leachates (pollutants which seep through the soil into the groundwater or streams) from tip sites and septic tanks can also put nutrients into a waterway.
How much nutrients leach from an area depends a lot on the type of soil. Coastal soils, such as the deep grey sands, don't hold nutrients very well. The nutrients go straight through into the groundwater. Sandy soils over clay are the next worst. Clay loam soils and brown and yellow sandy soils with a high iron content have the best ability to bind phosphorus and stop it from going through into the groundwater or seeping into streams.
Too many nutrients in a waterway can cause algal blooms. When the algae bloom they use up oxygen at night when they respire. Fish that live near the surface of the water where the algae are respiring might not have enough oxygen and become sick or die. There are other problems, too. When an algal bloom finally finishes and dies, this may result in deoxygenation of the water. Bacteria and other organisms start to eat the algae. When they do, they use up oxygen in the process. Again, fish and other creatures might not be able to get enough oxygen and might die. Also, anaerobic bacteria (bacteria which live where there's very little or no oxygen) in the bottom sediments may be released when oxygen levels are low. When this happens, the bacteria release other nutrients into the water which are usually stored in the sediment.
If there are a lot of nutrients in an estuary, some fish may not be able to live there. They may be stressed because they have too much or too little dissolved oxygen and they might go somewhere else (if there is anywhere for them to go) or they may die. They may also be killed if the algae is the poisonous, blue-green sort. Other fish and water creatures might flourish because they like the increased amounts of nutrients.
The algal bloom can also block sunlight from getting through to the plants on the bottom. If this continues for long, those plants can die.
| Total P | Total N | Nitrates | Nitrites | ||
|---|---|---|---|---|---|
| Aquatic ecosystems | 10-100 (a) | 100-750 (a) 5-50 (b) | > 400 (a) 100-500 (b) | no guideline > 250 (b) |
|
| Raw drinking water | no guideline | no guideline | > 50 | > 3 | |
| Irrigation | nutrients in water usually aid crop growth and are not considered pollutants unless present in excess | ||||
| Livestock | no guideline | no guideline | 30 | 10 | |
Soluble inorganic salts are the primary source of nutrients for the growth of phytoplankton (tiny waterway plants) and other plants, but they do not contain all the nitrogen and phosphorus available in the waterway. So it's most practical to use the total amount of nitrogen and phosphorus to work out the quality of the water.
To measure the total N and total P, a sample of water is taken. Suspended solids are not filtered out, because N and P both can be bound to suspended solids as well as being in solution in the water. Sample bottles are filled from the waterway, stored at 4°C and sent to a laboratory for analysis.
| Toxins are products which are poisonous to other organisms. |
Chemicals which can harm the estuary and its plants and animals are pesticides (insecticides and weedicides) and petroleum products. Plants and animals may be poisoned if these products are eaten or taken up by them. The animals or plants can become sick or die.
Toxins can be divided into two categories: direct and cumulative.
Direct toxins
These toxins have a direct, harmful effect on organisms. They include chemical products, such as pesticides, and biotoxins.
Pesticides are used to control a large variety of organisms which are considered pests. These include insects (insecticides), weeds (herbicides) and fungi (fungicides).
Biotoxins are microalgae that may occur naturally in the environment or have been introduced into the waterways from elsewhere. Two common biotoxins are blue-green algae and dinoflagellates. Blue-green algae is a type of algae that can release poisonous chemicals and change the pH of water from normal up to 11 (very alkaline). Dinoflagellates are microalgae which are toxic in their own right.
Cumulative toxins
These do not cause any direct problem until they have reached a particular level. They may be added to the water, the bottom sediments or be taken up by plants or animals over a period of time. Then illness or death of an animal or plant might be the result. These sorts of toxins are heavy metals, radiation and chemicals. If these products accumulate in plants or animals, there may be sublethal effects (not directly killing an animal or plant, but almost) such as birth defects in offspring, lesions on fish or thinning of the egg shells of birds. Radiation can cause cancer in some organisms.
Direct chemical toxins
These may enter the estuary because of accidental spillage straight into a waterway or through drains. When these things happen, there's usually a large amount of toxin, in concentrated form, put into the waterway. Direct biotoxins
These often naturally occur in the waterway. They can bloom when temperature, water clarity, nutrient level and salinity are right for them. Dinoflagellates may also get into a waterway from ballast water from ships visiting the port. Ships take in water (called 'ballast') on their journeys around the world to help keep the ship's weight in balance. When they get to a port they may release some of the water to allow heavier goods to come on board.
Cumulative toxins
These may come from a variety of sources. The toxins combine over many years to reach a level that affects the health of the estuary. Chemical toxins in this category may come from run off from parks and gardens and horticultural or agricultural land, leachate from tip sites and industry or from manufacturing processes.
Heavy metal contamination of the environment can come from:
Blue-green algae
When these algae are blooming they can block the gills of fish. This makes it very difficult for the fish to get oxygen from the water, and sometimes they can die. Some blue-green algae also release chemicals which cause poisoning and even death if they are eaten. They may also cause skin irritations in humans and to the gill filaments of fish. Other blue-green algae are known to change the pH of the water from a normal range of 7-8 up to a pH of 11. This can have a severe impact on animals and plants living in the vicinity, particularly invertebrates (such as shellfish and worms) which live in the sediments on the bottom and can't escape. Humans who eat filter-feeding organisms (such as shellfish) which have been exposed (because they couldn't get away from the area) to blue-green algal blooms may get paralytic shellfish poisoning.
Dinoflagellates
These are microalgae which are toxic in their own right. These organisms bloom under specific conditions and can also cause paralytic shellfish poisoning to people who eat shellfish which have been exposed to their blooms.
Pesticides
All pesticides change chemically once they're in the environment. Organophosphate insecticides are decomposed rapidly, while organochlorine insecticides break down slowly. So, organochlorines stay in the waterways much longer. How long a pesticide persists in a waterway depends on:
Chlorinated hydrocarbons
These insecticides are highly soluble in animal and plant fats (they stay in animals and plants easily) but weakly soluble in water (they don't stay in water long). Chlorinated hydrocarbons, because they store easily in plants and animals, can be found in tiny creatures and very large ones. They are easily transferred from one to another when an organism is eaten. Once the chlorinated hydrocarbons are eaten, an animal or plant can't get rid of them. The more chlorinated hydrocarbons they eat, the more there are in their body. How much residue is left in an organism depends on how much they took in, and for how long, the type of plant or animal, how the chlorinated hydrocarbons got into the organism (by being eaten or being drunk, for example), what type of pesticide is it that is carrying the chlorinated hydrocarbons, and how well the organism can change the poison in its body to something less harmful, or if they can excrete some of it. These toxic combinations of chemicals (called 'compounds') can cause organisms to reproduce less (called 'reduced fecundity'), can give them lesions (sores), cause a general heightened stress level and illness, or kill them.
Heavy metals
These toxins can create problems in waterway environments once they get into a plant or animal, too. Organisms can't get rid of them from their systems. Heavy metals can concentrate in waterway organisms up to 9100 times more than the surrounding environment's levels. Once they're in animals or plants, they become part of the whole web. Heavy metals may be evident in the liver, gills and pancreas of organisms and may lead to acute or chronic effects. These include lesions, reduced fecundity and general environmental stress in organisms, similar to pesticides.
Creatures that feed on leaves and plant matter (called 'detritus feeders') - including polychaete worms, prawns and fish such as mullet - may swallow heavy metals with the detritus they feed on. These metals can then become part of the whole food web, when other animals eat the detritus feeders and take the heavy metals into their bodies as well.
The Environmental Protection Authority has banned the use of antifouling paints, used on boat hulls, which contain a heavy metal product known as tributyl tin (TBT).
Water, soil, plants and animals are tested for pesticides and heavy metals (such as cadmium, mercury, lead, chromium, zinc, nickel, copper and cobalt). Plants and animals tend to accumulate pesticides and heavy metals in their bodies. Even if the toxins are in small amounts they can be detected. It is much harder to detect small quantities in water. The samples of plants and animals are analysed using standard methods in chemistry laboratories that produce consistently reliable results.
| A pathogen is a parasite which causes disease. |
Bacteria and viruses are the two types of pathogens which can harm an estuary. A virus is an organism, smaller than most micro-organisms, that multiplies using other living cells. Viruses cause disease. Bacteria are micro-organisms, neither animal nor plant, that play a large part in causing decay, putrefaction, disease and also help to fix nitrogen into the soil out of the air. Plants and animals, including people using the estuary, may get sick from the pathogens in the water. But it's rare for pathogens to be at levels high enough to cause disease.
Viruses
These may enter the waterway from animal effluent (in drains from piggeries, for example), leachate from tips and septic tank effluent into the groundwater, from sewage discharged directly into the waterway, and from run off from stock grazing areas.
Bacteria
Bacteria come from sources similar to viruses.
Pathogens can cause illnesses and even death to the organisms of the waterways and human beings. Human pathogens can endanger people in direct contact wih the water (for example swimmers or waterskiers), or people who eat contaminated filter-feeders such as as oysters (filter-feeders can concentrate bacteria and viruses).
Bacteria such as Escherichia coli, Streptococci spp. and Salmonella spp. in a waterway show that there is pollution by human urine and faeces, either from sewage leaching or draining into the waterway or people urinating and defecating directly into the water. Drinking or coming into contact with water with these bacteria can cause severe illness or even death.
Introduced pathogens can affect native plants and animals.
No single procedure is available that can be used to isolate and identify all pathogens.
| Chlorophyll 'a' is a measure of the amount of green pigment (chlorophyll) present in the water. |
This green colour is the photosynthetic material in microscopic algae called phytoplankton. High levels indicate that there are large growths of phytoplankton, fed by an excess of nutrients available in the water and/or the sediments. The water is eutrophic. Chlorophyll 'a' is commonly used to measure eutrophication. The amount of microscopic algae is also determined by counting cells. This also helps to identify the species of phytoplankton.
The basic classes of eutropy (or eutrophication) are oligotrophic, mesotrophic and eutrophic.
Oligotrophic (from the ancient Greek 'oligos', meaning 'few') waterbodies don't have much growing in them at all. There is not much algae and the water is very clear.
Eutrophic systems have a lot of algal blooms.
Mesotrophic systems are between the two above.
| Oligotrophic | < 4 |
| Mesotrophic | 4-10 |
| Eutrophic | > 10 |
Chlorophyll 'a' shows how much phytoplankton there is and whether there is eutrophication of the waterway. Chlorophyll 'a' doesn't have a direct effect on waterways. It just indicates the presence of phytoplankton.
All green plants contain chlorophyll 'a'. It makes up about 1% or 2% of planktonic algae when it's dry. Water samples with phtyoplankton in them are analysed in chemical laboratories to detemine the amount of chlorophll 'a'.
| Physical pollutants are objects in the water that have a harmful effect on the waterway. |
Physical pollution may come from litter being blown into the waterway or by people deliberately dropping it. One example is fishers throwing the wrapping from their bait into the waterway. Soil from erosion or in run off may enter the estuary, or dredging might bring up sediments from the bottom and make the water cloudy.
Litter and rubbish can harm animals if the animals swallow them or get caught up in them. Rubbish entering the waterway may also contain other pollutants which may spill or leach into the waterway (such as pesticides, cigarette butts or petrol cans).
Soil particles entering the waterway, or bottom sediments being disturbed, can float in the water. This may affect the gills of fish swimming in the area, reduce the clarity of the water (which can make it hard for birds to search for food in the water, for example, and also restrict the amount of light penetrating the water so that plants don't photosynthesise as much, which reduces the amount of oxygen in the water).
There is no standard method.